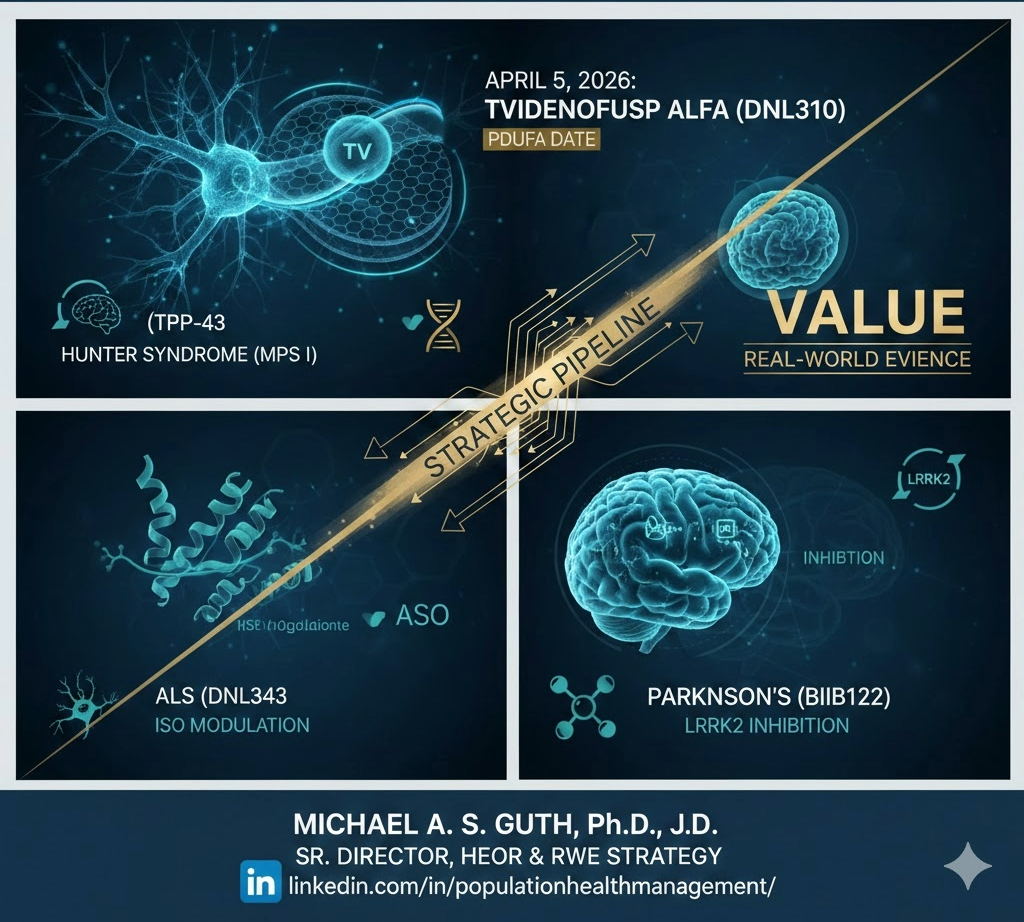
Beyond the BBB: Mapping the 2026 Milestone Year for Neuroscience. As we move through Q1, all eyes in the neuroscience community are on April 5, 2026—the PDUFA target action date for tividenofusp alfa (DNL310). If approved, tividenofusp alfa will be a watershed moment: the first commercial validation of a Transport Vehicle (TV) enabled enzyme replacement therapy designed to treat both the body and the brain in Hunter syndrome (MPS II).
But for those of us tracking the broader “Scientific Narrative,” the value of the TV platform extends far beyond lysosomal storage disorders. Denali is fundamentally rewriting the playbook for CNS delivery:
ALS (DNL343): Despite the recent HEALEY Platform trial missing its primary efficacy markers, the Integrated Stress Response (ISR) remains a critical biological target. The real-world evidence gained on serum NfL and ISR modulation is essential for refining how we treat TDP-43-driven pathology.
Parkinson’s (BIIB122): The focus on LRRK2 inhibition (currently in the Phase 2b LUMA and Phase 2a BEACON studies) represents the pinnacle of precision medicine in PD. Using urine BMP and blood pS935 LRRK2 as biomarkers isn’t just “good science”—it’s the foundation of a robust value demonstration for future payers. From a regulatory/HEOR perspective, the biomarker strategy here is as important as the efficacy data.
Success in neurodegeneration isn’t a straight line; it’s a series of engineering pivots. Whether it’s crossing the BBB or identifying the right patient subpopulations, 2026 is the year the industry sees if these “Molecular Architects” can bridge the gap between clinical signal and commercial reality.
#Neuroscience #HEOR #RWE #DenaliTherapeutics #ALS #Parkinsons #PDUFA #RareDisease #BiotechInnovation



 Posted in
Posted in